Beijing Duheng for Drug Evaluation&Research Co., Ltd. (DDER) a highly customer-oriented consultancy providing regulatory affairs and clinical support services, has a proven-track record of offering turnkey solutions that include local and global regulatory and clinical support coverages, for life-sciences companies in area of pharmaceuticals, medical devices, in vitro diagnostics, cosmetics, health food, and so on.We have successfully guided clients from all over the world, to oversee the complicated National Medical Products Administration (NMPA) registration process and bring the cutting-edge pharmaceuticals, medical devices and other healthcare products to the Chinese market, and in the meanwhile assisted the local customers to obtain the marketing authorization in the highly-regulated markets globally for the products manufactured in China.
Look to us for the full-spectrum services, proven expertise, access to competent authorities and industry leaders, and help from network affiliates to navigate your products from initial regulatory consulting and entry-into strategy design, through regulatory approval, and ultimately to the market of China.
SERVICE
Medical device and IVD registration in China:Medical device/IVD registration and regulatory compliance; NMPA certificate composition strategy ; Technical dossier preparation and on-site type testing support;Software and cybersecurity document complied to meeting NMPA requirement; NMPA application submission and communication with reviewers; Registration inspection coordination; NMPA certification renewal and modification, Translation & Sample Importation Logistics。
Clinical Research:Clinical trial assessment;Clinical trial protocol design;Sample size;Hospital & ethical committee review & approval;Clinical trial auditing;Monitoring and ensuring data quality with CRC and CRA, data management & reporting, and statically analysis;Post-market clinical trial。
Customer Reviews
"I would like to know how you do it. As a strategic partner, your company has maintained a positive working attitude in the past cooperation and completed the work projects with high quality. In the past cooperation, your professionalism is better than We have worked with any other CRO company, which is why we hope to work with your company for a long time.”
Head of a pharmaceutical company
Continue to innovate
Looking to the future, we are committed to the risk management of new drug innovations to help the healthy growth of the pharmaceutical industry!
Quality comes first
We strictly abide by the ethics and regulations of clinical research and development, strive for survival by quality, with a scientific, rigorous and truth-seeking professional attitude, and provide patients with safe products through standardized, high-quality and efficient clinical research-related services.
Share opinion
Du Heng Zhidao will regularly share industry insights and the latest information with you, so that you can get to know us and get more information and resources to accelerate clinical research.
Click on the title in the flowchart to learn more
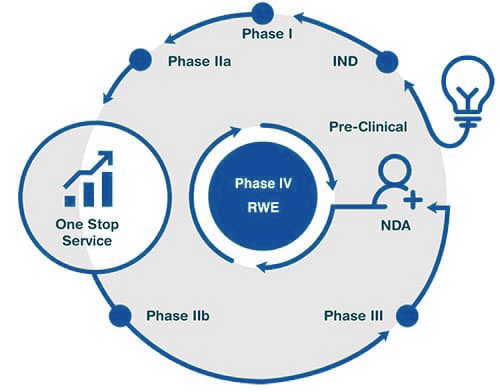
We use reliable technology and best practices to provide data support and solutions for your drug development decisions, helping you quickly move your products from the laboratory to the clinic.
• Early research and development
• Domestic and foreign registration and application of drugs, instruments, health food, special medical food, etc
• Domestic and international clinical trials
• Large variety breeding after market
• Third-party audit
More
All clinical studies on new drugs after they are approved for marketing by drug regulatory authorities are in the scope of post-marketing studies. In contrast to phase II and III clinical trials, the primary purpose of post-marketing clinical studies is to further monitor the safety and efficacy of drugs in an expanded population.
More
• Our team works closely with you to tailor your research to maximize efficiency and reduce risk. For phase I clinical trials, we leverage high-level trial design, advanced biometrics, excellent PK/PD experts and extensive project management experience to provide a complete solution that ensures rapid and high-quality data collection, analysis and reporting that will ultimately benefit your clinical development program.
• Thanks to our ability in biostatistics, we can use highly flexible study protocol design to help you obtain the clinical evidence you need and avoid unnecessary investment later. In addition, with a dedicated recruitment center and team, we can help you quickly find suitable subjects for your program.
More
• Our team works closely with you to tailor your research to maximize efficiency and reduce risk. For phase I clinical trials, we leverage high-level trial design, advanced biometrics, excellent PK/PD experts and extensive project management experience to provide a complete solution that ensures rapid and high-quality data collection, analysis and reporting that will ultimately benefit your clinical development program.
• Thanks to our ability in biostatistics, we can use highly flexible study protocol design to help you obtain the clinical evidence you need and avoid unnecessary investment later. In addition, with a dedicated recruitment center and team, we can help you quickly find suitable subjects for your program.
More
We use reliable technology and best practices to provide data support and solutions for your drug development decisions, helping you quickly move your products from the laboratory to the clinic.
• Drug metabolism and pharmacokinetics (DMPK)
• Toxicology services guide new therapies from discovery to full development
• A full range of bioanalytical services
• Pharmaceutical research helps your prescription enter clinical studies
• Bioequivalence studies to support local and international customer registrations for NMPA in China and US FDA generics
More
The increasing size of clinical trials has placed greater demands on study design, enrollment, and expertise in related therapeutic areas. We will tailor our solutions to meet your R&D needs using experienced clinical operations staff and advanced PK/PD and biometrics technology.
With a strong quality management system, sound standard operating procedures and rich experience in international project management, we have successfully completed many large clinical studies with high efficiency and low cost.
More
The increasing size of clinical trials has placed greater demands on study design, enrollment, and expertise in related therapeutic areas. We will tailor our solutions to meet your R&D needs using experienced clinical operations staff and advanced PK/PD and biometrics technology.
With a strong quality management system, sound standard operating procedures and rich experience in international project management, we have successfully completed many large clinical studies with high efficiency and low cost.
More
With rich project experience, I am able to fully understand the specific requirements of regulatory authorities around the world for application materials, accurately grasp the key points of product registration, and maintain effective two-way communication with customers and pharmaceutical administration departments from multiple levels and aspects such as procedural provisions, regulatory requirements and technical compliance to ensure the rapid progress of the project.
More